Biacore is based on surface plasmon resonance (SPR) technology to track real-time interactions between biomolecules in their natural state, without the need for any markers. It utilizes the principle that incident light can resonate with the plasma on the metal surface during total reflection to detect whether there is interaction between biomolecules and the dynamic parameters of the reaction. This technology has been widely applied in various fields such as immunology, proteomics, drug screening, protein nucleic acid interactions, and has obtained many kinetic data that cannot be obtained by other methods.
In 1983, Liedberg et al. from the Applied Physics Laboratory of LINKOPING Institute of Technology in Sweden first applied surface plasmon resonance technology to detect the interaction between IgG and its antigen, and developed an SPR sensor by Biacore company. Subsequently, the research and improvement of SPR sensors have rapidly developed, and their applications in biomedical fields have become increasingly widespread.

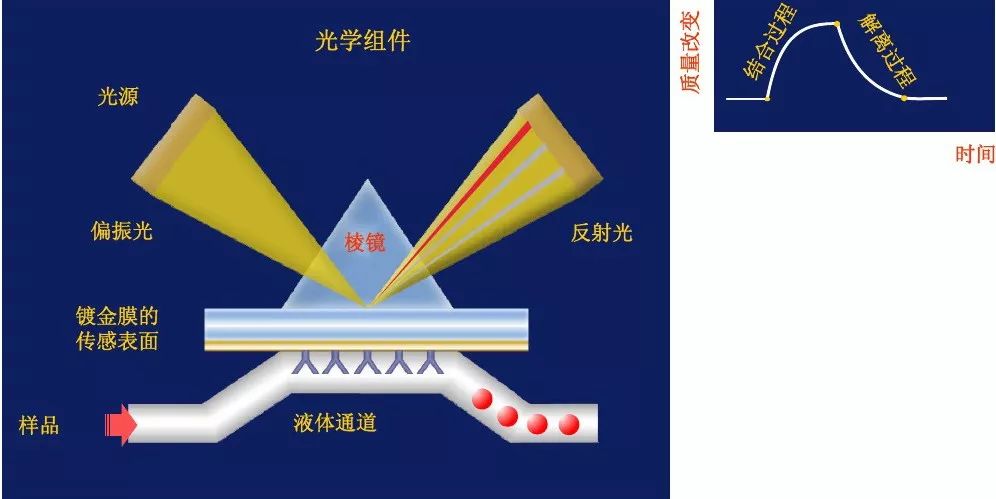
Surface plasmon resonance (SPR) is an optical phenomenon in which a layer of approximately 50nm thick metal film is present at the total reflection interface of a sensing chip. Polarized light is incident on one end of a prism, and surface plasmon waves are generated at the interface between the prism and the metal film. When the propagation constant of the incident light wave matches the propagation constant of the surface plasmon wave, free electrons in the metal film will resonate, known as a surface plasmon resonator.
When analyzing, first couple a biomolecule or ligand (protein, antibody, etc.) onto the surface of the biosensor, and then inject a solution containing another biomolecule (analyte) that can interact with the target molecule and flow through the surface of the biosensor. The binding between biomolecules causes an increase in the surface mass of biosensors, leading to changes in refractive index. By monitoring the angle changes of SPR, the dynamic binding and dissociation constants, affinity, and specificity of the analyte can be automatically obtained. The changes in the reactions between biomolecules are observed.
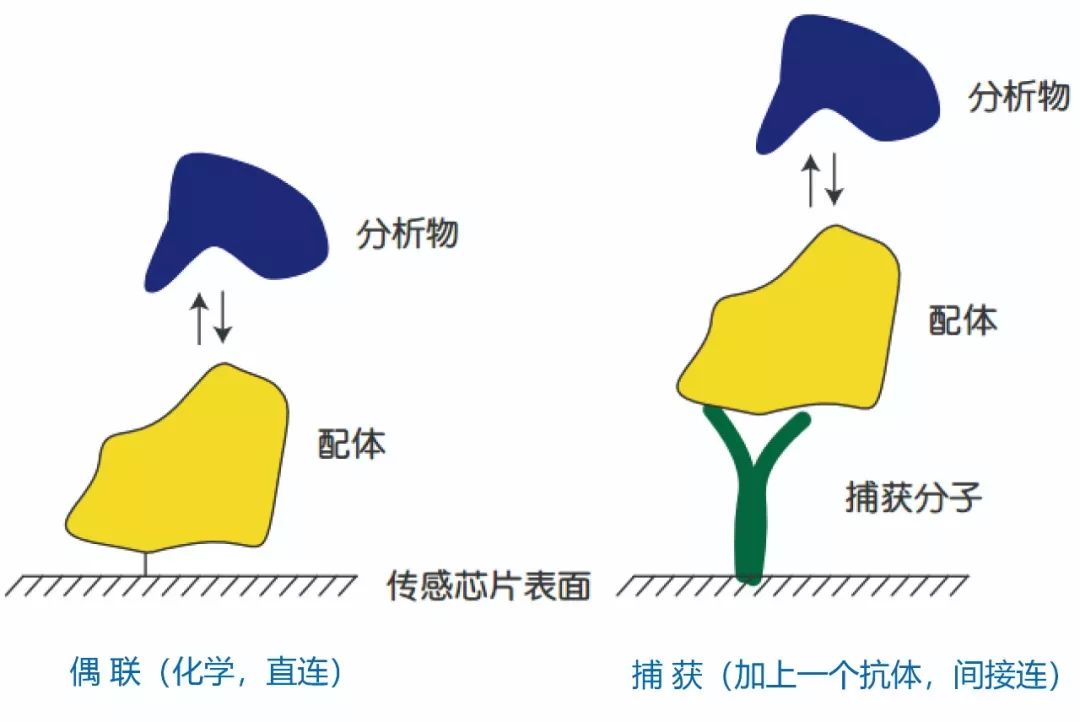
The direct coupling method utilizes the chemical groups of the ligand itself to covalently fix the ligand directly and ihheversibly on the chip surface. According to the characteristics of various ligands, different sensing chips and coupling methods can be selected for covalent coupling.
The capture method involves coupling capture molecules, such as anti mouse secondary antibodies, onto the surface of a chip, which can be used for batch analysis of the interactions between various mouse derived antibodies and antigens. Compared to the traditional ELISA assay used in antibody production to analyze antibody potency, the Biacore capture method can obtain more accurate and detailed screening results more quickly, simply, and at a lower cost. In addition to anti mouse secondary antibodies, cuhhent products for capturing molecules also include anti His tag antibodies, anti GST antibodies, anti human secondary antibodies, human Fab binding proteins, etc.
In contrast, the capture method has numerous advantages:
★ Easy to operate, shorten method development time
★ Directly capture hybridoma supernatant and ascites antibodies
★ Fast detection speed, each sample only takes 5-10 minutes
★ Lower operating costs and repeated use of chips
★ Can selectively bind ligands and enhance ligand activity
★ No need to explore coupling and regeneration conditions
★ Suitable for ligands with poor stability and sensitivity to acidity and alkalinity

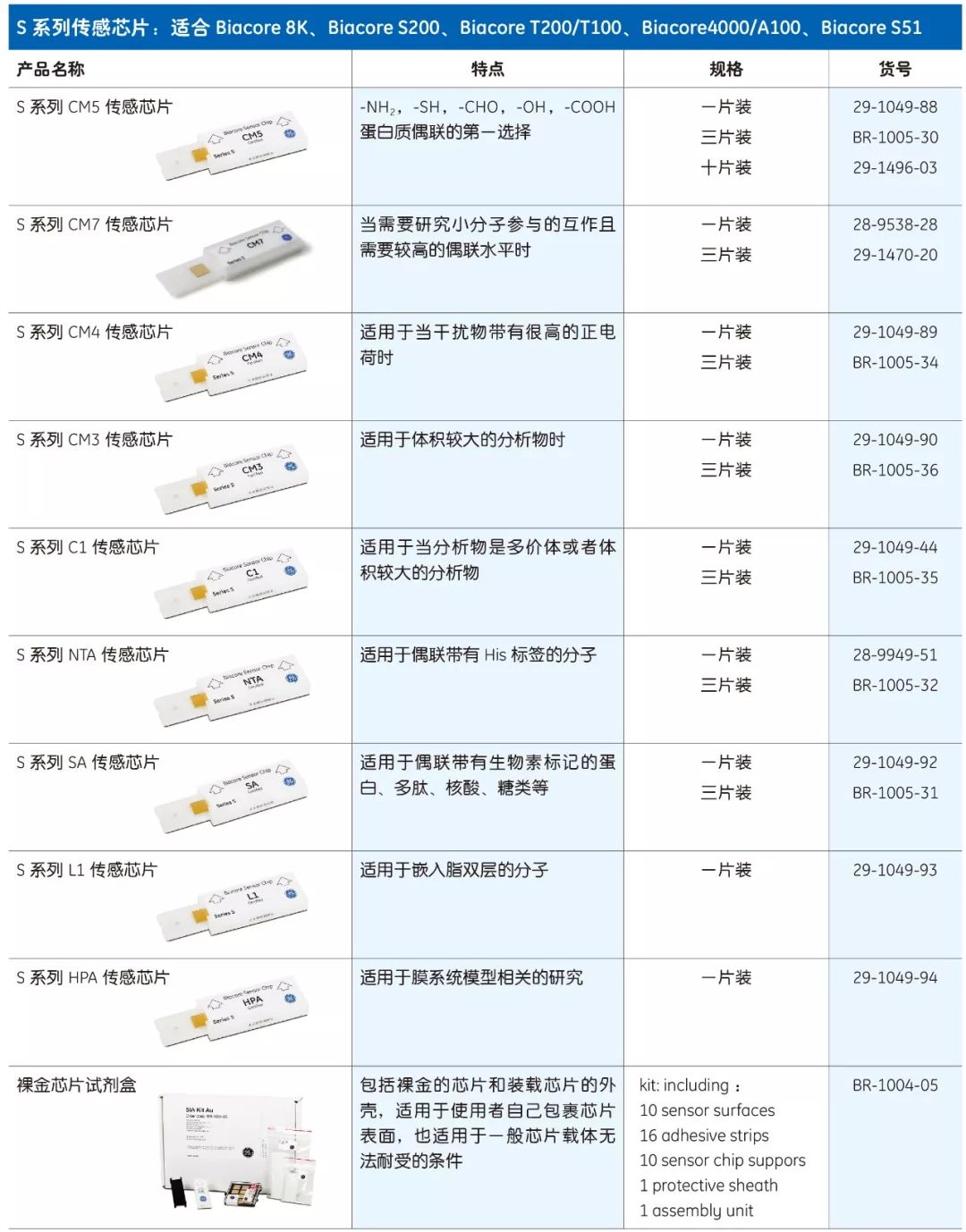
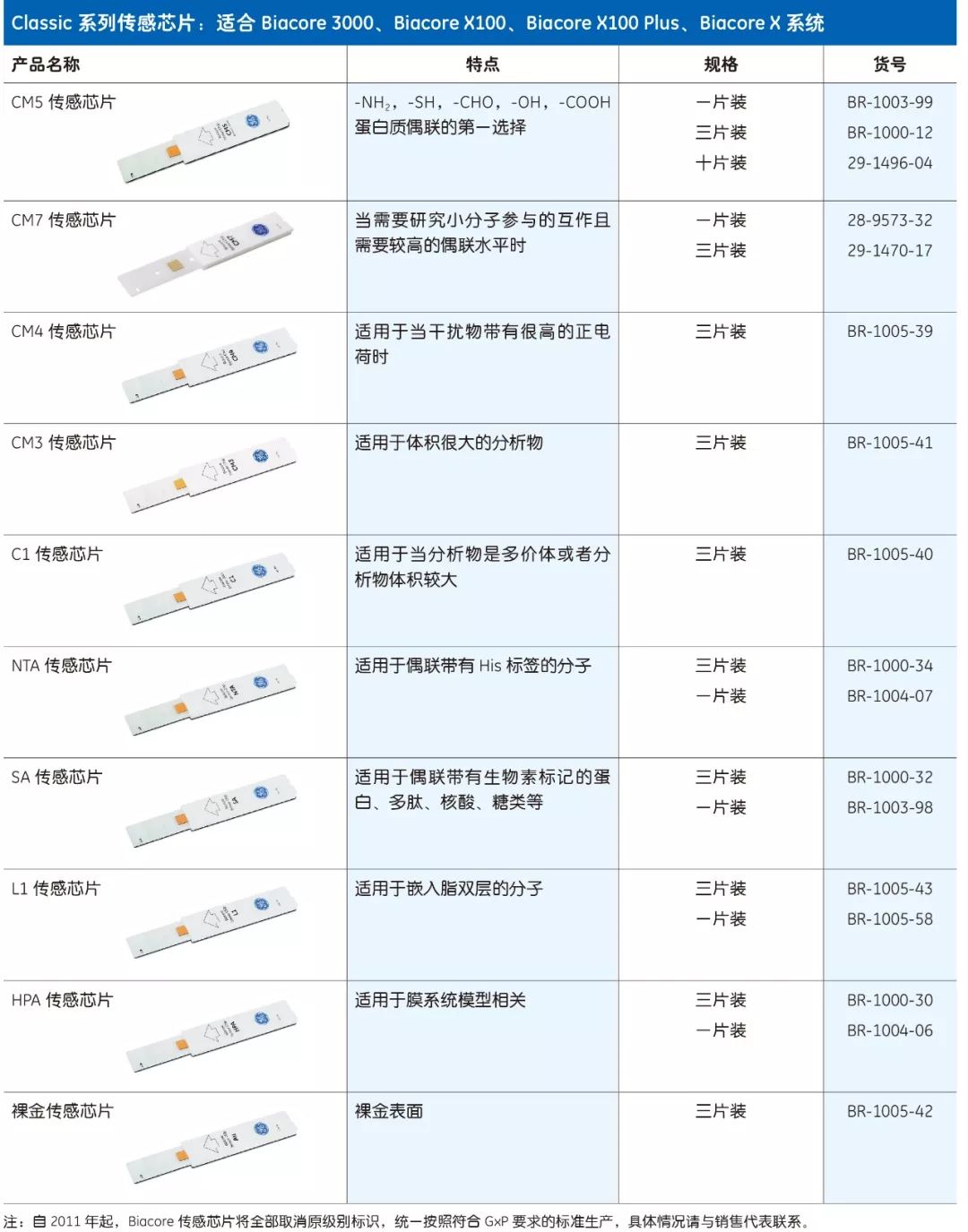
Biacore chips are divided into two series - Series S and Classic, each with 12 different chips. The only difference between the two series is the shape of the casing, which is suitable for different models of Biacore systems.
ordering information